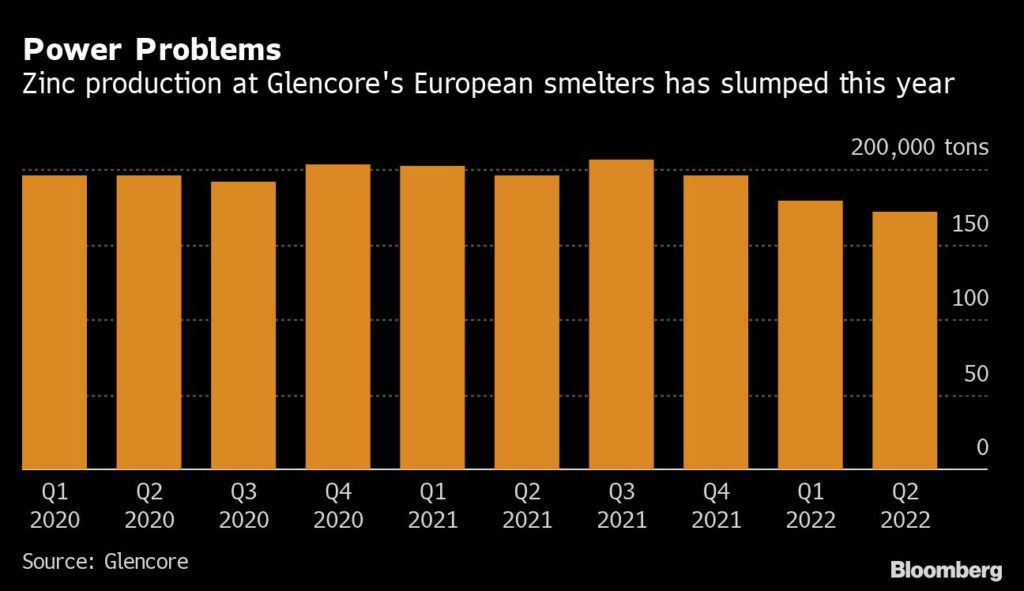
Mining
Monday, October 3rd, 2022 5:05 am EDT
Despite being commonly used, prior to this study, battery researchers had not really understood why nitriles work in the way they do.
In a paper published in the journal Nano Research, the Tsinghua group explains that lithium cobalt oxide (LiCoO2, or just ‘LCO’) is the most widely used material for cathodes in lithium-ion batteries, particularly those installed in portable electronic devices thanks to LCO’s high operating voltage, high capacity and stability. But what has made LCO cathodes particularly attractive is that they offer more energy for a smaller amount of space.
However, at the moment, LCO cathodes can only deliver roughly half of their theoretical specific capacity. This means that they often find it difficult to meet the increasing energy-density demands of portable electronics.
One way around this issue would be to increase the limited charge voltage of batteries using LCO cathodes. The limited charge voltage describes the point at which a battery is considered to be completely charged.
Beyond this point, the battery could be harmed, and so many devices just shut off when the cut-off threshold is hit. This threshold is somewhat arbitrarily set, and so it could be increased, and such high-voltage operation does offer promise for giving an energy density boost to such batteries.
But if this is done, a new problem arises: high voltage operation can exacerbate harmful chemical reactions at the interface between the cathode and the electrolyte. This can result in cracks and other internal damage, which in turn can further deteriorate the interface between the electrodes. Ultimately, all of this can result in a radical decline in battery capacity and a sharp increase in safety issues. The working temperature in these devices can hit 45℃ due to their own heat generation, further shortening battery life.
For some time now, mixing in additional compounds to the electrolyte formula to tweak the reaction activity of the electrolyte at the interface between the cathode has proven to be a very effective strategy to decrease the electrolyte reaction activity.
There are a number of different compounds used as such electrolyte additives, but for lithium-ion batteries, nitriles are perhaps the most widely used.
So to truly understand the working mechanism of nitrile additives, the researchers took a common one, tridentate ligand-containing 1,3,6-hexane-tri carbonitrile (or HTCN).
More stable interface
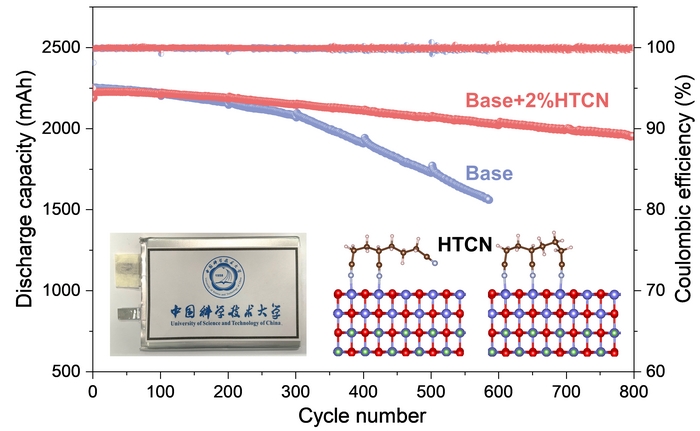
While the battery was operating, and far outperforming an equivalent without the HTCN electrolyte additives, the researchers used electron microscopy to have a closer look at what was happening. They combined their observations with an analysis of the ion and elemental composition of the system. They found that the HTCN additive was very effectively inhibiting the generation of cracks and the dissolution of cobalt ions.
Then, using X-ray photoelectron spectroscopy and a series of theoretical calculations, they found that the HTCN molecules were being efficiently adsorbed on to (stuck to) the surface of LCO and embedded in the interface between the cathode and the electrolyte, which in turn greatly inhibit an oxidation reaction on the surface of the LCO, thereby preventing a continuous decomposition of the electrolyte.
Altogether, this amounts to a much more stable interface between cathode and electrolyte, which significantly suppressed the decomposition of components and the formation of cracks. It is this stable and dense cathode-electrolyte structure that enhances the stability of the battery through a great many cycles of charging and discharging.
Having this molecular-level understanding of how nitrile additives give such batteries a boost, researchers can now seek out other electrolyte additive formulations that produce a similar or better interface structure.
This post has been syndicated from a third-party source. View the original article here.